Help your patients adhere to BV treatment1 with
NUVESSA® —
The one and only single-dose intravaginal
metronidazole gel treatment for BV
Savings Program
Eligible patients may pay as little as $25b for their prescription
Get Started Now
bThe patient is responsible for the first $25 of their co-pay and cash-paying patients
should pay approximately $55. For cash paying patients or
insured patients when
NUVESSA is not covered by primary insurance, patients may still use this savings card but
may have an outstanding balance.
Please see Program Terms, Conditions, and Eligibility Criteria on back of card or here.

Prescribe NUVESSA® and deliver the assurance of the gold standard metronidazole in one targeted dose at bedtime.
Not all metronidizole-containing prescription products are the same2:
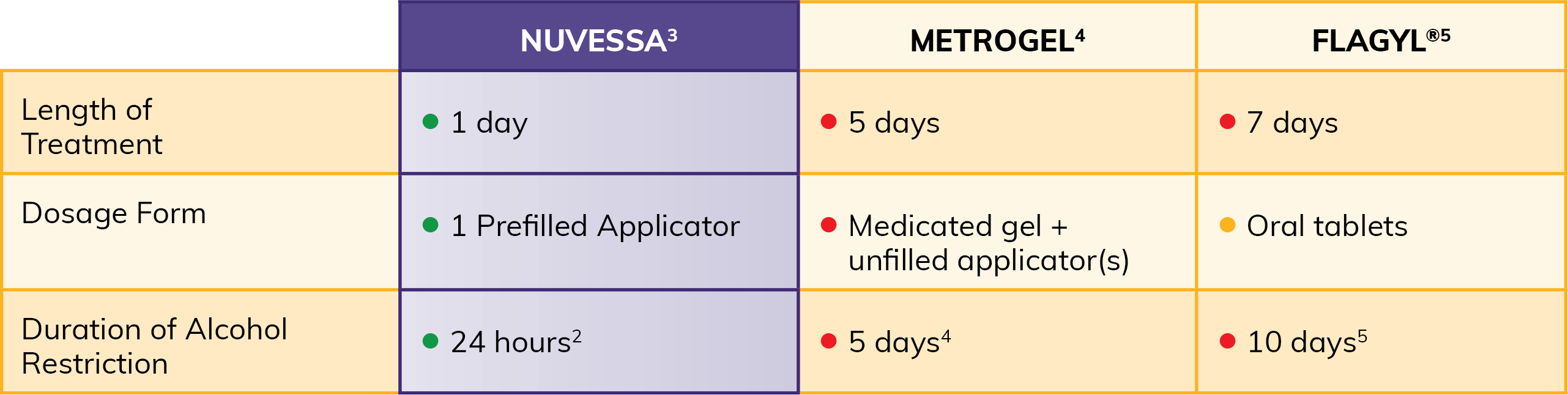
When used as directed, a single bedtime dose of NUVESSA® avoids adherence issues associated with multi-dose treatments.1
Deliver efficacy with single-dose adherence
- Pre-filled applicator for her convenience3
- Lower systemic exposure (intravaginal administration results in 2-4% of the systemic exposure a single, oral dose of metronidazole 500-mg oral tablets)3
Why prescribe 5 doses when you can prescribe 1?
A 1-time dose at bedtime has been proven effective against bacterial vaginosis (BV)1
The safety and efficacy of Nuvessa was assessed via a single, randomized, double-blind, vehicle-controlled clinical trial among subjects with a clinical diagnosis of bacterial vaginosis in order to evaluate the efficacy of Nuvessa.
The primary endpoint in this phase 3 study was clinical cure at Day 21, defined as return of
physiologic vaginal discharge, confirmed by the
investigator with a negative whiff test and clue cells < 20%(with no pH criterion).
In addition, secondary endpoints included bacteriologic and therapeutic cure at Days 7 and 21; clinical cure at Day 7, and time to resolution of symptoms.

NUVESSA® Outcomes in Placebo-Controlled and Comparative Clinical Trials
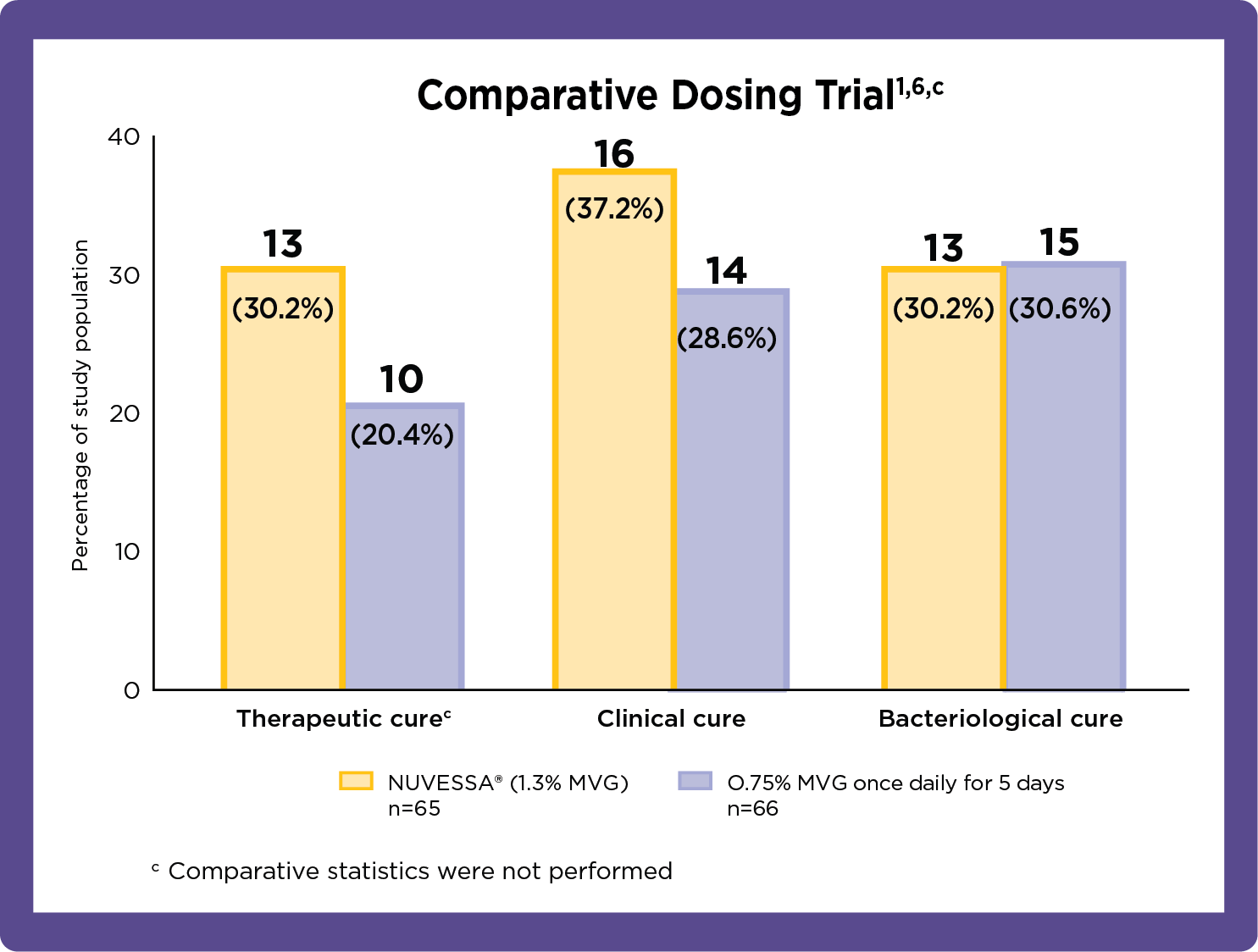
The primary endpoint in this phase 3 study was therapeutic cure (defined as
clinical plus
bacteriologic cure) at Day 21.
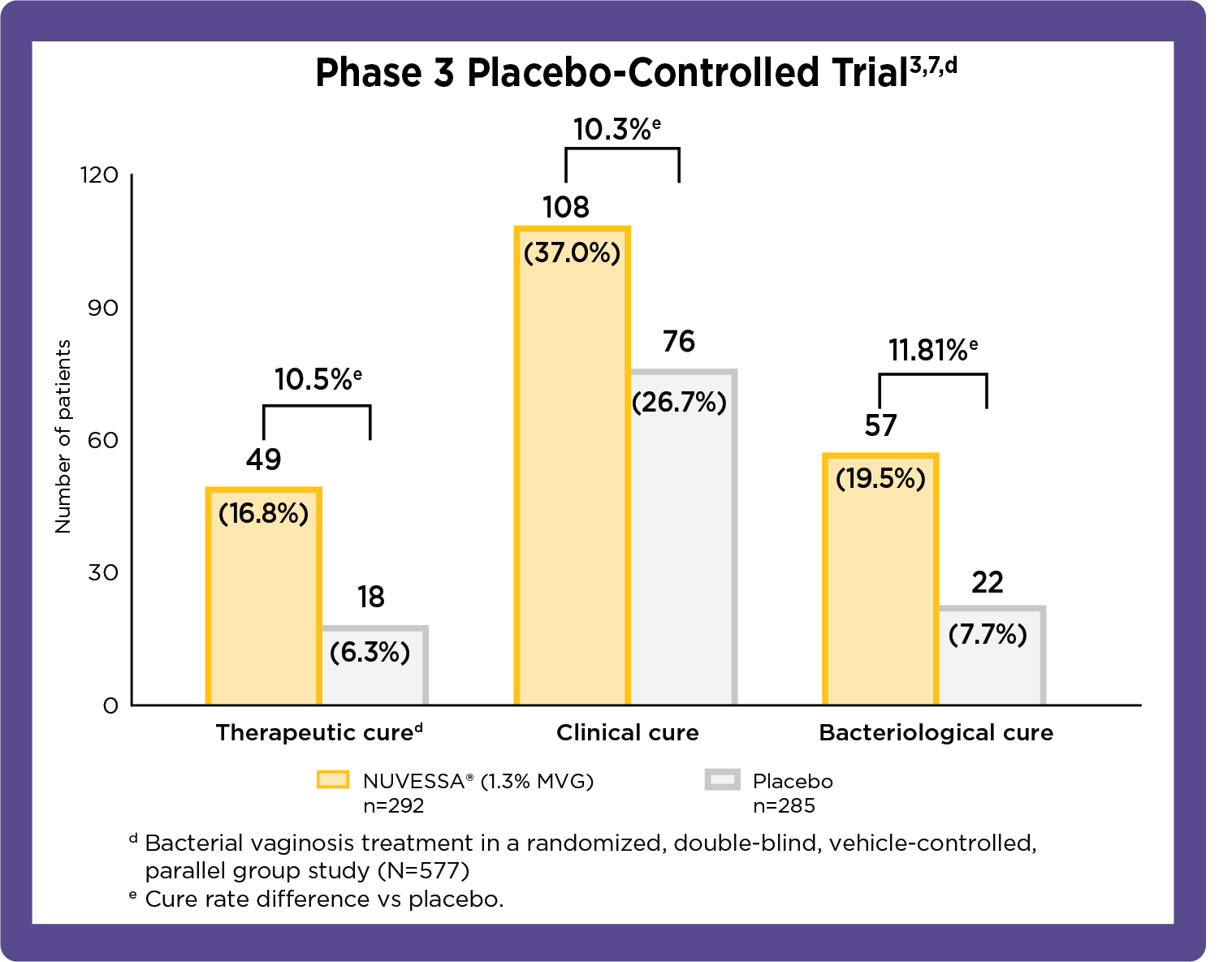
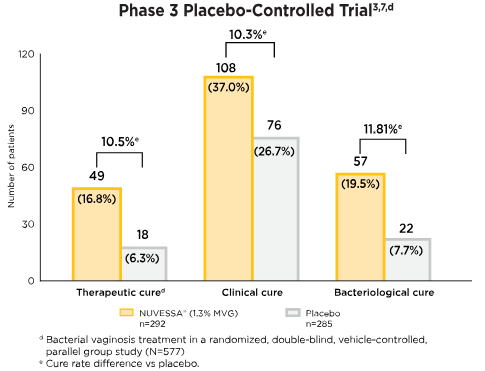
Cure rates at test of cure/end of study: per-protocol1
Therapeutic cure implies patient's infection resolves in response to medical treatment. Clinical cure is based on investigator assessment in clinical trials against specific end points. Bacteriological cure requires both the elimination of the infection and restoration of normal vaginal microbiome.8
- At day 7, Clinical Cure was achieved by a statistically significantly greater proportion of patients in the NUVESSA group vs. the vehicle gel group (41.1% vs. 20.0%). Bacteriological Cure was also achieved by a statistically significantly greater proportion of NUVESSA-treated patients vs. the Vehicle Gel group (33.9% vs. 6.3%).3
- The safety of NUVESSA was evaluated in a randomized, double-blind, vehicle-controlled study in subjects with bacterial vaginosis. There were no deaths of serious adverse reactions. Adverse reactions were reported by 19.0% of NUVESSA-treated subjects vs 16.1% of Vehicle Gel-treated subjects.3,f - Adverse reactions occuring in >1% of subjects receiving NUVESSA were: vulvovaginal candidiasis (5.6%), headache (2.2%), volvovaginal pruritus (1.6%), nausea (1.6%), diarrhea (1.2%), and dysmenorrhea (1.2%). No subjects discontinued treatment due to adverse reactions.
f321 non-pregnant females with a mean age of 33.4 years (range 18-67 years) received NUVESSA. Subjects administered a single dose of NUVESSA at bedtime on the first day of the study.
Help your patient adhere to BV treatment1 with NUVESSA® — the one and only single-dose intravaginal metronidazole gel for BV
- 1 dose of 1.3% MVG contained in NUVESSA® delivers the efficacy of 5 doses of 0.75% MVG1
- 1 pre-filled applicator offers ease of use at bedtime3
- 1 targeted dose at the site of infection minimizes unnecessary drug exposure6


To ensure your patients receive NUVESSA®, add “Dispense as written”
Make sure your patients get the only single-dose, single-use metronidazole vaginal gel 1.3% treatment for BV

INDICATION AND IMPORTANT SAFETY INFORMATION
IMPORTANT SAFETY INFORMATION
CONTRAINICATIONS
NUVESSA is contraindicated in:
- Patients with a history of hypersensitivity to metronidazole, parabens, other ingredients of the formulation, or other nitroimidazole derivatives.
- Patients using disulfiram or within 2 weeks of disulfiram use.
- Patients using alcohol. Alcoholic preparations (e.g., ethanol, propylene glycol) and beverages should not be consumed during and for at least 24 hours after NUVESSA therapy.
WARNINGS and PRECAUTIONS
Central and Peripheral Nervous System EffectsConvulsive seizures, encephalopathy, aseptic meningitis, optic and peripheral neuropathy have been reported in patients treated with oral or intravenous metronidazole. NUVESSA should be administered with caution to patients with central nervous system diseases. Discontinue promptly if abnormal neurologic signs development.
Carcinogenicity in AnimalsMetronidazole has been shown to be carcinogenic at high doses administered orally in mice and rats. Unnecessary use of metronidazole should be avoided.
Drug/Laboratory Test InteractionsMetronidazole may interfere with certain serum chemistry values, such as aspartate aminotransferase (AST, SGOT), alanine aminotransferase (ALT, SGPT), lactate dehydrogenase (LDH), triglycerides, and glucose hexokinase. Values of zero may be observed.
Adverse ReactionsThe most common adverse reactions (≥1%) observed in adult clinical studies were vulvovaginal candidiasis, headache, vulvovaginal pruritus, nausea, diarrhea, and dysmenorrhea. The most common adverse reactions (≥1%) observed in pediatric clinical studies was vulvovaginal discomfort. This is not a complete list of risks.
INDICATION
NUVESSA® (metronidazole vaginal gel 1.3%) is an intravaginal treatment indicated for the treatment of bacterial vaginosis in females 12 years of age and older.Please see full Prescribing Information here.


